 |
| Home | Current Issue | Archives | Bookshelf | Online Features | Marketplace | Subscribe |
| In This Section |
| Site Search |
 |
|
Welcome, Anil Somayaji
Sigma Xi Member
|
DOI: 10.1511/2003.2.122
Influenza
The world is teetering on the edge of a pandemic that could kill a large fraction of the human population
|
It has all the makings of a cheesy Hollywood horror flick: A shape-shifting killer travels the globe, leaving millions of corpses in its wake, and the world's medical community can't stop the carnage. It's a sophomoric idea for a movie script, but that's exactly what unfolded during the waning months of the first World War, late in 1918, and through much of 1919. Within 10 months the influenza virus affected the lives of up to 500 million people across the globe and killed at least 20 to 40 million—more than twice the number who died on the battlefields of World War I. Many epidemiologists believe that a similar scenario will happen again. But this time it will be worse. This is not hyperbole. In 1997 the world came perilously close to another global epidemic of the "flu." If this particular virus had attained the ability to spread from person to person, the pandemic might have taken the lives of a third of the human population. As it was, only six people died—and all of them had contracted the virus from chickens sold in Hong Kong poultry markets. The only thing that saved us was the quick thinking of scientists who convinced health authorities to slaughter more than a million domesticated fowl in the city's markets. The avian virus turned out to be a new strain—one that the human population had never seen before. These deadly new strains arise a few times every century, and the next one may arrive any day now. Most of us are reminded of influenza every autumn when the medical community invites the public to receive the annual "flu shot," or when we succumb to a mild form of the disease during the winter months. Symptoms typically include fever, chills, sore throat, a lack of energy, muscular pain, headaches, nasal congestion and a suppressed appetite. But the flu can quickly escalate, prompting bronchitis, secondary infections, pneumonia, heart failure and, in many cases, death. Infants, the elderly and people with suppressed immune systems are at highest risk of dying from the flu. People who have serious conditions such as lung or cardiovascular disease are also in danger. The exception to these risk factors occurred in the 1918 "Spanish flu" pandemic, when almost half of the people who died were between the ages of 20 and 40. It's still not clear why previously healthy people in this age bracket had such high mortality rates.
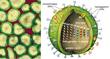
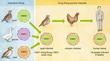
Lesser influenza pandemics took place in 1957 (the "Asian flu") and 1968 (the "Hong Kong flu"). There were also flu "scares" in 1976 (the "swine flu") and in 1977 (the "Russian flu"). Precisely how and when the influenza virus will develop into an extremely pathogenic form is beyond our current ability to predict. We understand the virus's structure, how it enters the cells of the human body and how it evades detection by the host's immune system, but knowing these things is not enough to stop another pandemic. The issues extend beyond science into the realms of international and local politics, national budgets, and deeply entrenched cultural traditions. The purpose of this article is not to instill fear, but to educate—the more people there are who understand the problems, the more chance we will have to contain the next outbreak. In-Flew-EnzaInfluenza is spread from person to person by coughs and sneezes, but the virus doesn't begin its journey in a human host. Instead, wild aquatic birds such as ducks and shore birds perpetuate the influenza viruses that cause human pandemics. Although these birds carry the genes for influenza in their intestines, they usually don't become sick from the virus. And because they can migrate thousands of miles, the healthy birds can spread the virus across the globe even before the microbe makes contact with the human population. As it happens, the form of the virus found in wild birds doesn't replicate well in human beings, and so it must first move to an intermediate host—usually domestic fowl or swine—that drinks water contaminated by the feces of aquatic birds. Horses, whales, seals and mink are also periodically infected with influenza. Although the intermediate hosts can sicken and die from the infection, swine can live long enough to serve as "mixing vessels" for the genes of avian, porcine and human forms of influenza. This occurs because swine have receptors for both avian viruses and human viruses. Swine have probably played an important role in the history of the human disease. These animals appear to serve as living laboratories where the avian and mammalian influenza viruses can come together and share their genes (a reassortment of RNA segments) and create new strains of flu. When a strain of virus migrates into the human population, it changes into a disease-causing microbe that replicates in the respiratory tract. A sneeze or a cough spreads the virus in a contagious aerosol mist that is rich in virus particles. Most pandemics originate in China, where birds, pigs and people live in close proximity. Hong Kong's 1997 "bird flu" was an avian influenza virus that probably attained virulence through reassortment of genes from geese, quail and teal. Many bird species were housed together in the Hong Kong poultry markets, and this was an ideal environment for reassortment. This strain of influenza killed thousands of chickens before it moved to human beings. Eighteen people were infected—all through direct transmission from chickens, not from contact with other people. In this instance, the outbreak was curtailed before the virus could mutate into a form that could spread from person to person. Scientists had known since 1972 that the influenza virus originated in aquatic birds, but the 1997 epidemic was the first case to document influenza's direct transference from poultry to people. Anatomy of a Killer
Influenza viruses are members of the Orthomyxoviridae family, and they fall into one of four genera—A B, C and thogotovirus, which is a tick-borne virus. Type C influenza does not seem to cause serious disease. The type B virus, recently isolated from seals in Holland, often creates regional epidemics in human populations. But type A influenzas have avian lineages, and these are the viruses that cause human pandemics. The influenza virus contains eight separate RNA segments that encode genes for at least 10 proteins. This unusual genetic structure explains why reassortment happens so often. If two different viruses infect the same cell, an exchange of gene segments can easily take place, yielding up to 256 (or 28) different offspring.
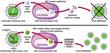
Type A influenzas are categorized by the structural variations of two glycoproteins, hemagglutinin (HA) and neuraminidase (NA), which protrude from the surface of the virus. HA's job is to attach the influenza virus to the sialic acid receptors on the surface of the human cell. After binding, the flu virus penetrates the host cell; there, viral RNA strands move into the cell's nucleus. The viral RNA strands encode messenger RNA and ultimately produce new virus particles. The task of NA is simple but important: It enables the newly created virus to separate from the host cell and travel freely from one cell to another through the respiratory tract. Scientists have identified 15 HA and 9 NA subtypes, all of which are found in avian hosts. Epidemics occur when the HA or the NA proteins mutate. The subtypes of type A viruses are named according to the particular variants of the HA and NA molecules they contain—such as H1N1, the culprit in the 1918 holocaust and the 1976 "swine flu" scare, or H5N1, the "bird flu" of 1997. Influenza's unpredictability springs from its ability to alter its HA and NA surface proteins and so avoid identification by the host's immune system. When a person is infected with the flu, the immune system produces antibodies and cell-mediated responses against all of the virus's gene products (antigens). If the person later encounters the same virus, his antibodies will bind to it and prevent an infection. However, the virus can alter antigenic sites—points on the HA and NA molecules where the antibodies would normally bind—by the process of antigenic drift. In DNA-based genomes a proof-reading enzyme carefully scrutinizes the process of copying a strand of DNA, catching and correcting any mistakes made during replication. But, like other RNA-based viruses, the influenza virus lacks a proofreader, so mistakes made during replication go uncorrected and the virus can mutate swiftly. The mutations can change the antigenic sites in such a way that the host's antibodies no longer recognize the virus. The HA and NA molecules are particularly important in antigenic drift. As genetic point mutations are gradually accumulated by the viral genome, and the HA or NA genes and proteins have undergone several minor changes, the host's antibodies no longer recognize them, and the person may sicken again. Type B influenza strains use this process to alter the amino acid structure of these proteins and so evade the human immune system. Every 20 to 30 years or so, the type A influenza virus undergoes an antigenic shift. If antigenic drift were compared to a shudder, antigenic shift would be likened to an earthquake. Antigenic shift engenders a much more immediate and dramatic change in the HA glycoprotein. During antigenic shift, genes from other influenza subtypes can completely replace the HA and NA proteins with new ones that the host has never experienced. When human immune systems cannot recognize the new virus, a pandemic ensues. Scrutinizing the ShiftInfluenza was first described by Hippocrates as early as 412 b.c., and the tiny virus has spent the succeeding centuries shifting, drifting and wreaking havoc. Humanity has been seeking ways to eliminate the threat since the first pandemic was recorded in 1580. Although the Spanish flu happened nearly a century ago, the extreme pathogenicity of the H1N1 1918 influenza virus is still not understood. Virologists have traveled the world to obtain samples of the virus so that they could unlock the secrets of its virulence, even exhuming victims from the Alaskan and Norwegian permafrost. Jeffery Taubenberger and his colleagues at the Armed Forces Institute of Pathology studied bodies and fragments of lung samples that had been stored in paraffin blocks since 1918. Through sequence and phylogenetic analysis of RNA fragments taken from the lung tissues, they determined that the virus was avian in origin but was closely related to a strain of influenza that is known to infect swine. Ongoing studies of the total genome sequence may someday uncover reasons for the potency of this strain of influenza. If they can understand the genome of the virus and the genome of the host, scientists may be one step closer to pinpointing which viruses in the wild will cross over to human beings. When the H5N1 virus leapt from poultry to people in 1997, scientists from the World Health Organization (WHO) immediately began to investigate the phenomenon. Postmortem examinations of two victims revealed unusually high levels of cytokines—proteins such as interferon and tumor necrosis factor-alpha (TNF-a)—that regulate the intensity and duration of the immune response. These cytokines are the first line of defense against viruses. They are part of the innate immune response, a nonspecific response that will target any pathogen and does not require a previous exposure to a virus (unlike the production of antibodies, which does require the exposure to viral antigens). Studies of human macrophage cultures, by Malik Peiris and his colleagues in Hong Kong, show that the H5N1 virus causes an exaggerated response of cytokines (such as TNF-a), and this could result in a toxic-shock-like syndrome (including fever, chills, vomiting and headache), which ultimately results in death. Although the cytokines can sometimes inhibit its proliferation, the virus may develop strategies to subvert this innate immune response. This is what happened in Hong Kong. The H5N1 virus found a way to circumvent the effects of the infection-fighting cytokines. Sang Seo, Erich Hoffmann and author Robert Webster at St. Jude Children's Research Hospital used reverse genetics—the opposite of the traditional gene-to-protein direction of genetic analysis—to identify a gene that played a crucial role in the transformation of the influenza virus. The new technology offers many tantalizing opportunities: It might drastically reduce the time required for vaccine production, and it might help scientists gain insights into viral pathogenicity. We removed the so-called nonstructural (NS) gene from H5N1 and inserted it into a previously benign strain of flu. Experiments showed that the newly transformed virus was considerably more virulent in swine. Pigs infected with a virus that carried the NS gene experienced much more severe and prolonged fever, weight loss and viremia than pigs that were not infected with a virus containing that gene. This suggests that the product of the NS gene, the NS1 protein, plays a crucial role in limiting the antiviral effects of the cytokines. According to Adolfo García-Sastre and Peter Palese of the Mount Sinai School of Medicine, and their colleagues, the NS1 protein seems to do this by downregulating the expression of genes involved in the molecular pathway that signals the release of the cytokines.
Gene sequencing reveals that a single point mutation occurred in the NS gene of the Hong Kong virus. This changed the identity of an amino acid—glutamic acid at position 92 in the NS1 protein—which produced a version of the protein that was much more effective at downregulating the activation of the cytokines than the normal version. This made the Hong Kong virus much more virulent than other influenza viruses—a remarkable consequence for such a tiny alteration. These discoveries might help us to understand the extreme pathogenicity of the 1918 influenza virus, and they perhaps suggest new targets for drug development. In 2001 a new variety of the H5N1 virus surfaced in the live poultry markets of Hong Kong, but this time the fowl were slaughtered before people could become infected. Yet another genotype of H5N1 appeared in 2002. This evidence indicates that viruses similar to the 1997 strain are still circulating in the bird population of Southeast China. They are reassorting and making new versions of H5N1—not the same virus that surfaced in 1997, but different mutations that retain the same HA and NA configurations. Prevention and TreatmentThe changeable nature of the influenza virus ensures that it can escape immune surveillance and circumvent the body's defense mechanisms. Moreover, the influenza vaccine that protected humans against infection last year may be ineffectual this year. Scientists at more than 100 WHO laboratories are constantly collecting and analyzing the influenza viruses that circulate in the human population worldwide. After isolating the viruses for antigenic and molecular analysis, WHO scientists annually identify two type A strains and one type B strain that are most likely to cause epidemics during the coming season. Vaccine manufacturers then incorporate all three strains into the vaccine composition that will be used for that year. The resulting flu shots protect individuals only from the targeted strains—not from unexpected viruses that may arise after the WHO determination has been made. Years ago, influenza vaccines were impure, whole-virus vaccines that caused the recipients to run fever and display other flu symptoms. Most pharmaceutical companies today split the virus into subunit vaccines, which contain only specific viral protein units. To create the vaccine, technicians grow the vaccine virus in fertile hens' eggs. The virus is then inactivated (so that it cannot cause infection) and purified. Because these vaccines are not made of live viruses, but only purified portions of those viruses, immunization promotes immunity but does not cause infection. The human immune system then creates antibodies that attack viruses containing those proteins. Several other types of vaccines are also in development. Some evidence suggests that weakened live-virus vaccines may prompt a more protracted immune response than subunit vaccines. Recent clinical trials by Robert Belshe of St. Louis University School of Medicine and William Gruber of Vanderbilt University have indicated that a new nasal spray containing such a live-virus vaccine is safe and effective in both children and adults. DNA vaccines and vaccines created through the use of reverse genetics may also prove useful someday.
The classic way to create a seed virus for production of an influenza vaccine is to generate a virus that contains six genes from a high-yield virus such as H1N1 and two genes (HA and NA) from circulating strains. This method of creating a seed virus is cumbersome and time-consuming. Recently, however, scientists at St. Jude discovered how to generate the high-yield virus using eight plasmids (laboratory-made molecules of double-stranded DNA, which is made from viral RNA). This eight-plasmid system allows for the rapid generation of reassortment influenza A viruses, which can be used as master virus seeds for the manufacture of vaccines. Another goal is to find ways to produce vaccines more quickly. When a pandemic occurs, pharmaceutical companies must manufacture a vaccine as quickly as possible, while they incorporate procedures to ensure that the drugs are both safe and effective. The time required to produce, test and distribute a new flu vaccine ranges between seven and eight months, so it's virtually impossible to produce an adequate amount of vaccine during a pandemic. In 1976, laboring in the shadow of an expected "swine flu" pandemic, American drug manufacturers produced 150 million doses of vaccine—enough for the entire U.S. population. Given today's increased population and stringent regulatory processes, vaccine production might take much longer. A number of groups are seeking ways to drastically reduce that production time. Flu outbreaks generally pose the most serious threat to people who are very young, elderly, immunosuppressed or chronically ill. In the United States, vaccination is suggested for people who are at least 50 years old or deemed to be at high risk for infection. Canada is a little more progressive—in Ontario, vaccinations are available at no charge for citizens older than 6 months. People infected with the flu have a high risk of dying from bacterial pneumonia. Pneumococcal pneumonia kills thousands of elderly people in the United States each year. During an influenza pandemic, this mortality rate skyrockets. Vaccines are now available to protect people against almost all of the bacteria that cause pneumococcal pneumonia and other pneumococcal diseases. For years, antiviral drugs such as amantadine and rimantadine occupied the front line of influenza treatment. These drugs obstruct the function of an ion-channel protein called M2. When taken during exposure to influenza, these M2 inhibitors may help prevent infection, and if infection has already taken hold, their early administration may reduce the severity and the duration of the symptoms. But because type B influenzas do not possess M2 molecules, the drugs are effective only against type A influenza. More important, all strains of influenza quickly acquire resistance to these drugs. Two families of antiviral drugs have been developed that are less prone to resistance, have fewer adverse side effects than M2 drugs, and are effective against types A and B influenza. These antineuraminidase drugs hobble the NA glycoprotein on the surface of the influenza virus. When NA is inhibited, the virus is unable to release itself from the host cell to spread infection—it simply gets stuck and dies. If administered soon after the initial infection, NA inhibitors such as zanamivir and oseltamivir can effectively prevent viral replication. Preparing for a PandemicWhen the H1N1 virus crossed the globe in 1918-19, physicians watched helplessly as their patients succumbed quickly to pneumonia and other complications of influenza. The suffering patients had no access to antibiotics, vaccines or antivirals. Today we live in a world where air travel is common. A tourist in Hong Kong can spread the virus around the globe within hours. Whether a pandemic comes about as a result of natural forces or bioterrorism, the world is currently unprepared for the onslaught.
Unfortunately, another pandemic is inevitable. Historically, pandemics sweep the globe several times every century. Thanks to the efforts of the World Health Organization, scientists are conducting surveillance studies of the influenza virus at the animal-human interface. That surveillance probably prevented a worldwide catastrophe in 1997. Virologists in thousands of laboratories are trying to predict the virus's movements. By learning, for example, how one mutation in the "bird flu" helped that virus circumvent cytokine responses, they are a step closer to understanding influenza's evolutionary processes, and to developing drugs to combat the virus's effect. But even the most sophisticated methods and the latest discoveries offer no guarantee of predicting the next pandemic. Asia—particularly Hong Kong—has been identified as the epicenter for influenza pandemics. After the 2001 outbreak of H5N1 in Hong Kong, a new regulation was installed: All poultry must be removed from the markets on a specific day each month to minimize the chance of viral replication. A better solution to the problem would be to replace the live poultry markets with markets selling frozen or refrigerated meat. But the poultry markets are an integral part of the Hong Kong economy and its culture, so they aren't likely to be eliminated in the near future. Similar live-poultry markets in New York City should also be closed. Because of cultural mores, politics and entrenched traditions, however, the Hong Kong and New York markets will likely remain open until another pandemic erupts, forcing the issue. When a virus does manage to evade the scientific community's gatekeepers, it may travel the world in a matter of hours. Fewer than a dozen companies worldwide currently manufacture flu vaccine (in the U.S. there are only two companies making vaccine), and even though the influenza outbreaks of the past two years have been relatively mild, these companies have had difficulty meeting the demands for vaccine. Subunit vaccines take months to create, so vaccine manufacturers will be incapable of producing enough vaccines to subvert the progress of a pandemic. M2 inhibitors such as amantadine and rimantadine may be useful if the virus does not acquire resistance to their effects. The NA inhibitors offer the most promise for treatment options in the event of a pandemic, but they are expensive and in short supply. For NA inhibitors to be effective, they must be administered soon after the initial infection. Drug companies require a year and a half to produce adequate quantities of antivirals. Unless production and stockpiling of drugs begins well in advance of a pandemic, adequate supplies of antivirals will not be available.
WHO and the developed nations of the world have created pandemic plans that specify ways to prepare for a world crisis. In the United States, the plan includes measures for improving surveillance systems and increasing the breadth of the country's vaccination programs. The plan also supports research into detection of new strains and the creation of new vaccines and antivirals. The national pandemic plan addresses such topics as communication systems, as well as medical readiness and how community services will be maintained. One way to prepare for the inevitable pandemic is to vaccinate as many people as possible during the interpandemic years. No-cost, universal vaccine programs such as the one in Ontario offer perhaps the best way to increase the capacity to make a vaccine in a crisis. The nations of the world must develop these plans. If a pandemic happened today, hospital facilities would be overwhelmed and understaffed because many medical personnel would be afflicted with the disease. Vaccine production would be slow because many drug-company employees would also be victims. Critical community services would be immobilized. Reserves of existing vaccines, M2 inhibitors and NA inhibitors would be quickly depleted, leaving most people vulnerable to infection. The nations of the world spend untold billions on military equipment, stockpiling bombs and other weapons. But governments have not invested a fraction of that amount into stockpiling drugs for defense against influenza. The scientific community has a responsibility to convince nations to stockpile NA inhibitors and promote vaccine production. The cost to developed nations would be minuscule, compared with the social and economic disaster that will occur during a full-scale pandemic. AcknowledgmentsThe authors are grateful for the continued support of St. Jude Children's Research Hospital, the American Lebanese Syrian Associated Charities (ALSAC) and the National Institute of Allergy and Infectious Diseases. |
|
I had a little bird . . .
Nearly 500,000 people died of the flu in the United States from 1918 to 1919. In many cities, public gatherings were prohibited, livestock markets were closed and coffins were in short supply. American troops unwittingly participated in biological warfare as they carried the “Spanish flu” to the battlefields of Europe during the first World War. Forty percent of the healthy young Americans who shipped out for Europe succumbed to influenza rather than the flying bullets on the battlefield. In time, the enormity of this event was lost to our cultural memory. Back to top | ||||
Bibliography
- Belshe, R. B., and W. C. Gruber. 2001. Safety, efficacy and effectiveness of cold-adapted, live, attenuated, trivalent, intranasal influenza vaccine in adults and children. Philosophical Transactions of the Royal Society, Biological Sciences 356:1947-1951.
- Garca-Sastre, A., et al. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324-330.
- Hoffmann, E., S. Krauss, D. Perez, R. Webby and R. G. Webster. 2002. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine 20:3165-3170.
- Laver, W. G., and R. G. Webster. 1972. Antibodies to human influenza virus neuraminidase (the A2/Asian/57 H2N2 strain) in sera from Australian pelagic birds. Bulletin of the World Health Organization 47:535-541.
- Neumann, G., and Y. Kawaoka. 2001. Reverse genetics of influenza virus. Virology 287:243-250.
- Reid, A. H., T. G. Fanning, J. V. Hultin and J. K. Taubenberger. 1999. Origin and evolution of the 1918 "Spanish" influenza virus hemagglutinin gene. Proceedings of the National Academy of Sciences 96:1651-1656.
- Seo, S. H., E. Hoffmann and R. G. Webster. 2002. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nature Medicine 8:950-954.
- Seo, S. H., M. Peiris and R. G. Webster. 2002. Protective cross-reactive cellular immunity to lethal A/Goose/Guangdong/1/96-Like H5N1 influenza virus is correlated with the proportion of pulmonary CD8(+) T cells expressing gamma interferon. Journal of Virology 76:4886-4890.
- Simonsen, L., M. J. Clarke, L. B. Schonberger, N. H. Arden, N. J. Cox, and K. Fukuda. 1998. Pandemic versus epidemic influenza mortality: A pattern of changing age distribution. Journal of Infectious Diseases 178: 53-60.
- Taubenberger, J. K., A. Reid, T. Janczewski and T. Fanning. 2001. Integrating historical, clinical and molecular genetic data in order to explain the origin and virulence of the 1918 Spanish influenza virus. Philosophical Transactions of the Royal Society, Biological Sciences 356:1829-1839.
- Webby, R. J., and R. G. Webster. 2001. Emergence of influenza A viruses. Philosophical Transactions of the Royal Society, Biological Sciences 356:1817-1828.
- Wood, J. M. 2001. Developing vaccines against pandemic influenza. Philosophical Transactions of the Royal Society, Biological Sciences 356:1953-1960.
- Young, D., C. Fowler and K. Bush. 2001. RWJ?270201 (BCX-1812): a novel neuraminidase inhibitor for influenza. Philosophical Transactions of the Royal Society, Biological Sciences 356:1905-1913.
|
Of Possible Interest
Book Review: Kuru Culture
Feature Article: Pathogens, Host-Cell Invasion and Disease
Feature Article: New Antibiotics and New Resistance
Book Review: Smallpox, Then and Now
Feature Article: Survival of the Fittest Molecule
| ||
| Related Internet Resources | ||
| Related Sigma Xi Links |








 request classroom permission
request classroom permission